888
Views & Citations10
Likes & Shares
Chemotherapy in cancer treatment is considered a superior mode of therapy in view of the various cytotoxic drugs that target the cancer cells milieu; destroy the cancer cells; selectively degrade the proteins involved in cell survival; inhibit angiogenesis to a limited extent and modulate cancer cells apoptotic intracellular pathways and bring nearly complete elimination of cancer cells at the primary site as well as prevent their migration to the nodal regions, in patients diagnosed at stage I and II. Though metastatic cancer patients (III and IV) end up in palliative terminal care with drugs like opiates that relieve their physical and mental pain, with no other way of cure or rehabilitation is currently available, than to end up their lives peacefully and painlessly, rehabilitation of cancer survivors with cognitive impairment and cure is feasible by understanding the neuro-endocrine and metabolic basis, and treating by phytochemicals like quercetin, rutin, β-carotene, flavonoids, flavonols, catechins, etc. which have established their therapeutic potentials of neuroprotection and enhancement of learning and memory and cognition abilities through rat model studies and by studies on human subjects suffering functional disorders of brain due to HPA axis activity and its sequelae.
Keywords: Neuroendocrine, Chemotherapy, Phytochemicals, Cognitive impairment, Cancer cells
INTRODUCTION
Among all cancer treatment type chemotherapy represents the most successful mode of therapy as it has improved the survival period for a long term. Such long term survival in cancer patients was found to be higher in women with breast cancer (23%) followed by prostate cancer individuals (19%) and then by (10%) of the individuals with colorectal cancers. However the above prolonged survival period is determined by several factors besides the detection of cancer stage at I and II levels, viz. [1],
1. The impact of post-treatment period diets and supplement
2. Regular exercise strategy
3. Stress free psychostatic condition
4. Sleep
5. Post treatment medication type
6. Immune competence, etc.
CIPN
Chemotherapy induced peripheral neuropathy(CIPN) is an important side effect due to various drugs such as taxanes, platinum agents , epothilones and vinca alkaloids, etc., and their cumulative toxicity. The severity of CIPN has been attributed to single drugs used for long times, multiple drugs and their combined toxicity, dosage regimens and intervals between successive dosage regimens etc. Although the treatment to these individuals with drugs has been conducted, there are no reports of established cure for the CIPN, in cancer survivors.
A larger percentage of cancer survivors reported memory A larger percentage of cancer survivors reported memory and concentration impairment. 20-30% of breast cancer and colon rectal cancer survivors showed cognitive impairment. The various domains in cognitive impairment include failure in working memory, deficits in executive function, information processing speed and memory retrieval, etc. [2]. In cancer survivors unlike the cortical deficits characteristics of age related Alzheimer’s dementia, the CIPN, related dementia involves sub cortical impairment which is reversible and treatable. Thus cognitive rehabilitation is a very important aspect for the survivors from cancer and is one of the strategies in rehabilitation programmes.
Harvey et al. [7] have also reported the selective neurotoxic action of P-chloro-mphetamine in the brain of rats with changes like neural cells shrinkage; perineuronal space and cellular debris, etc. Toth et al. [8] have reported reduction of forebrain weight with no gross lesions by chlorine dioxide in developing rat brain. The above studies opined that the changes in brain are likely events to happen since blood brain barrier regions and some zones in situ may allow the free passage of toxicants and/or biocides like chlorine compounds and pesticides. Balasundaram et al. [9] in their study on phosalone poisoning on the cation linked ATPases of CNS of Rana tigrina revealed failure of neuronal activity by the LD50 concentration (3.953 g/Kg body weight) of the O.P. pesticide. These authors revealed the inhibitory action of phosalone on the Na+ K+- ATPase; Mg2+, ATPase and Ca2+ ATPase; invariably in all the six regions of CNS, viz., rhombencephalon, lumbar spinal cord, cervical spinal cord, thoracic spinal cord, midbrain and telencephalon.
Effect of uncouplers on brain
It is known that uncouplers are compounds which can inhibit phosphorylation in cellular mitochondria but stimulate respiration and ATP hydrolysis [10]. Uncouplers in appropriate concentrations can prevent the phosphorylation of ATP without interfering the electron transport [11]. Pesticides like dinitrophenol derivatives, penta-chlorophenol and hexachlorophene and organophosphates can act as uncoupling agents interfering the oxidative phosphorylation but enhancing the O2 uptake in cells [11].
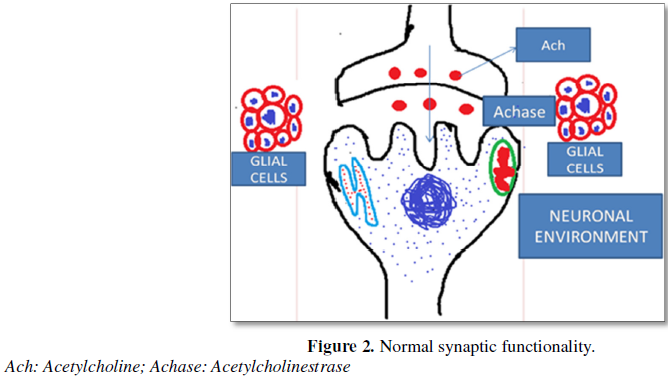
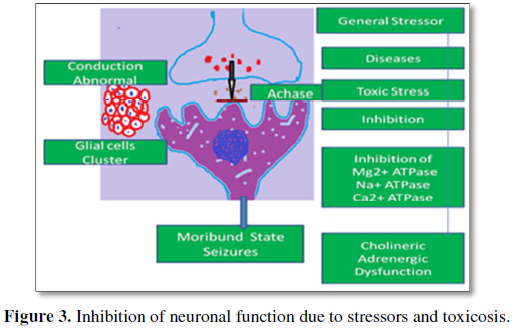
Balasundaram et al. [9] have also envisaged that inhibition of Ca2+ ATPase cause accumulation and enhancement of intracellular calcium due to efflux of Ca2+ from mitochondria as well as influx of Ca2+ from the external environment of the neural cells and may cause continuous release of neurotransmitter molecules and subsequent convulsions. Balasundaram and Selvarajan [12] have reported inhibition of ACHase (Acetyl cholisnesterase) and attributed disturbance in adrenergic and cholinergic functionalities leading to fatigue and moribundity in Rana tigrina.
The above informations from toxicological studies and stress biology provide us more insights regarding cognitive impairment in cancer survivors. That is a plethora cellular/neural mechanisms may operate to destabilize the functionalities of the different brain regions in cancer survivors and bring homeostatic disturbances, in order to manifest their cognitive abnormalities.
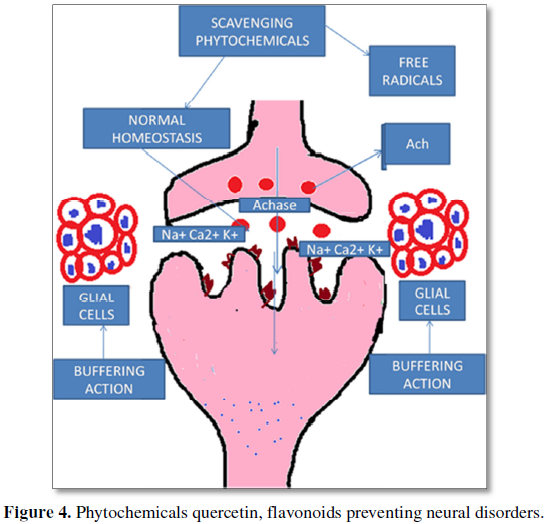
Several phytochemical compounds exhibit neuroprotective effects, Quercetin (Qc): is a polyphenolic compounds found in vegetables, fruits, seeds etc. In situ model studies have revealed that quercetin can pass through the blood-brain barrier [13]. Studies have also revealed that it significantly protected the neuronal cells from the oxidative stress induced neurodegeneration in Alzheimer’s disease [14], decreased the lipid peroxidation by the improved activity of catalase and superoxide dismutase and the glutathione depletion [15].
The herbal medicine Ginkgo biloba containing the Querectin in high amount exhibits neuro-protective effect against the oxidative damage inflicted by 6-OHDA [16]. It also attenuated the neuronal death in the hippocampus and improved the learning and memory in rats. Its flavonoids are mainly responsible for cognitive health of brain as they enhance the oxygen intake in brain. Sriraksa et al. [17] in their study on rat model with Quercetin suggest the use of Quercetin as an adjuvant therapeutic agent for the treatment of cognitive impairment.
The plant Nelumbo nucifera used in traditional medicine for various diseases originating due to free radicals injury and inflammation of the tissues revealed an array of bioactive compounds of different chemical groups, viz., alkaloids, flavonoids, glycosides, triterpenoids, vitamins and minerals. Its leaves, rhizomes, flowers and seeds exhibit antioxidant, anti-inflammatory and immune modulatory effects. In this context, the immune modulatory effect is so vital to cancer survivors. A report about the medicine of cancer revealed that people who lived near Three Mile Island nuclear plant (USA) at the time of accident had developed cancers after 3 years not because of excess radiation exposure but because of their excess cortisol level and reduced immune response due to trauma and stress. Similarly individuals who were more anxious and pressured by their work in military academy and who were engaged in stressful activity revealed fewer disease Fighting T-Lymphocytes in their blood than expected [18]. Similarly in cancer survivors their immune system may be less responsive due to treatment stress and the latent effect of stress continuum may have caused the cognitive impairment due to their past psychological stress.
The phytochemical molecules are known to enhance the antioxidant enzymes and non-enzymic antioxidants to reduce the free radicals which are the basis and cause for tissue inflammation and cellular damage of all metabolic tissues including brain. Our personal laboratory findings that beta-carotene ameliorated the aortic aneurysms in pathological liver and spleen or Apo-E knockout mice, also suggests that phytochemical in compounds invariably can alleviate tissue damages including cerebral aneurysms [19-21], as well as, they can set right the neuronal excitement, and cognitive impairment due to stressors.
Basis of cognitive impairment due to stressors in cancer survivors
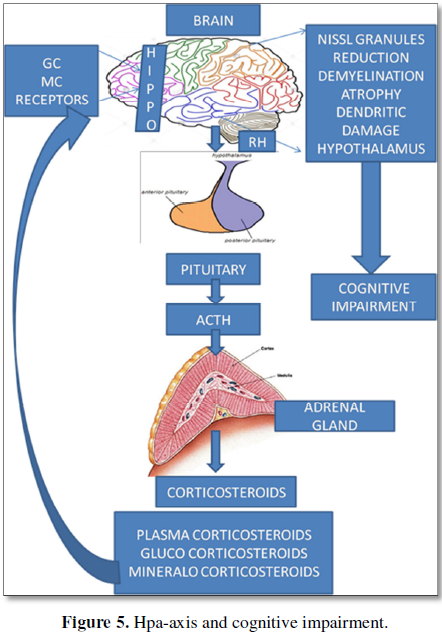
In stressor induced feedback of HPA axis and loop, the mechanism of HPA axis operation is determined by the presence and absence of stressor to either to open up the loop or to shut it down and return to the homeostatic point. The above HPA axis activity in cancer survivors may be associated with various adverse events during their treatment periods and may be continued as a regular phenomenon event after remission, showing the symptoms of cognitive impairment. Recent investigations have revealed that chronic exposure to stress hormones during gestational development, by the mother (maternal stress) will have its impact on HPA axis activity in the children starting from six months to five years and/or ten years [22-24].
Such gestational influence of maternal stress have a bearing in the neurological and cognitive behavior of the children and may reflect in their adult hood, in manifold forms such as unsocial behavior, sleep disorders, some psychiatric drug abuse, mood swings and anxiety disorders, etc. [25]. In these situations, the treatment with drugs could be expected to bring only partial recovery and the long lasting effects of earlier exposures to stress may continue to exist and operate. The impairment in cancer survivors may thus be conceived to be the effects of a repertoire of metabolic events and substrates, HPA axis activity, glucocorticoids levels, functionalities of such regions as Hippocampus, Amygdale, Dendritic profiles, Glucocorticoid Receptor (GR) mRNA levels in the pre frontal cortex of brain, and other factors like the inflammatory cytokines, and the metabolic hormones etc. Hence it is evident from these studies on development that stress exposure either prenatally or postnatally at child hood or at adolescence or at adulthood or at aged level may trigger the stress reactions in a continuum. The exhibits of all cognitive impairment behaviors in cancer survivors are also due to such stress reactions that occurred during their treatment periods since the time of detection until remission.
In this context, the literature on vitamin supplements like that of Vit-D or D3 remains inconclusive in regard to its action on brain functions [26]. However plant based natural nutrient/chemical molecules have been established in recent research, as apt anxiolytic compounds to function as stress busters as well as restoratives of the various brain functions and disorders in their own right.
Considering the stress evoked HPA axis activity and its continuum in their prenatal and post natal periods of development and also until adulthood in Human the disease implications with reference to cognitive impairment may have paternal - maternal - progeny bearing and metabolic basis alongside neuroendocrine axis. Such instances are not uncommon with reference to other diseases also like muscular dystrophy. For instance Milhorat and Goldstone [27] have reported that healthy fathers of dystrophic children had elevated serum creatine phosphokinase and lower body potassium and thus revealed the carrier state in male carriers. Similar to the above Blahd et al. [28] have reported decreased body potassium in non-dystrophic relatives of patients with muscular dystrophy. These authors have also reported that mothers with normal serum creatine phosphokinase gave birth to dystrophic offspring.
In yet another report by Blahd et al. [28] and Ramalingam et al. [29] revealed that 5 out of 10 mothers of dystrophic progeny and 6 out of 13 healthy female siblings had lower total body potassium. These earlier studies also preclude the possibility of the presence excessive HPA activity in the cancer survivors, and also in their family history. Such continuum of stress based cognitive impairment may be attributed if not to all cancer survivors; it may be applicable or probable in familial cancer survivors. The identification of genomic signals in such survivors brain cells genome and that of Adrenal cells which are responsible for the switch ‘on’ and switch ‘off’ of cortisol/corticosteroid production could be of therapeutic significance for their cognitive impairment management through phytochemicals molecules which have been found to inhibit certain pathways which cancer cells exploit for their proliferation and also enhance the genes to promote apoptotic pathways [29].
CONCLUSION
1. ASCO (2009) American Society of Clinical Oncology. Educational book, Orlando, Florida.
2. Vardy J, Rourke S, Tannock IF (2007) Evaluation of cognitive function associated with chemotherapy: A review of polished studies under recommendation for future research. J Clin Oncol 25: 2455-2463.
3. Brooks AS, Seagert GL (1978) The effect of intermittent chlorination on ten species of warm water fish. Centre for great lakes studies. The Univ. Wis. Milu. Miluaukee. Wis, p: 1.
4. Zeitoun IH, Reynolds JZ (1978) Power plant chlorinantion. Environ Sci Technol 12: 780-783.
5. Meier JR (1990) Mutagens in chlorinated water: Mutation and the Environment. Part E. Wiley-Liss Inc., New York, pp: 11-19.
6. Ramalingam K (2003) Chlorine toxicity on the brain histology of Oreochromis mossambicus (Treave) - Environmental quality assessment. Biochem Cell Arch 3: 81-85.
7. Harvey JA, Memaster SE, Yunger LM. (1975). Chloromphetamine: Selective neurotoxic action in brain. Science 187: 841-843.
8. Toth GP, Long RE, Mills TS, Smith MK (1990) Effect of chlorine dioxide on the development rat brain. J Toxicol Environ Health 31: 29-44.
9. Balasundaram K, Ramalingam K, Selvarajan VR (1995) Phosalone poisoning on the cation-linked ATPases of central nervous system of Rana tigrina (Daudin). Comp Biochem Physiol 111: 451-455.
10. Neil MW (1968) Invertebrate biochemistry. Pitman Med Publishing Co.: London.
11. Corbett JR (1974) The biochemical mode of action of pesticides. London: Academic Press.
12. Balasundaram K, Selvarajan VR (1990) Inhibition of acetylinesterase in the central nervous system of Rana tigrina by an organophosphate. J Biochem Toxicol 5: 65-66.
13. Youdium KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ (2004) Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 36: 592-604.
14. Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K et al. (2003) Parkinson’s disease is associated with hippocampal atrophy. Mov Disord 18: 784-790.
15. Fiorani M, De Sanctis R, Menghinello P, Cucchiarini L, Cellini B et al. (2001) Quercetin prevents glutathione depletion induced by dehydrosacorbic acid in rabbit red blood cells. Free Radic Res 34: 639-648.
16. Ferro MM, Bellissimo JA, Anselmo-Franci MEM, Angellucci NS, Da Cunha C, et al. (2005) Comparison of bilaterally 6-OHDA-and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurological, motor and memory alterations. J Neurosci Methods 48: 78-87.
17. Sriraksa N, Wattanathorn J, Muchimapura S, Tiamkao S, Brown K, et al. (2012) Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid Based Complement Alternat Med.
18. Ridley M (2002) Genome: The autobiography of a species in 23 chapters. Fourth Estste tld: Harper Collins Publishers Ltd., p: 344.
19. Gopal K, Nagarajan P, Jedy J, Raj AT, Gnanaselvi KV, et al. (2013) β-carotene attenuates angiotensin II-induced aortic aneurysm by alleviating inflammatory macrophage recruitment in Apoe-/-mice 8:e67098.
20. Gopal K, Nagarajan P, Shankar EM, Kumar JM, Kamarul T (2014) High-fat diet and Aniotensin II induced aneurysm concurrently elicits splenic hypertrophy and β-carotene ameliorates splenic pathology in Apoe-/-mice. Eur J Clin Invest 44: 1169-76.
21. Gopal K, Gotham M, Sachin Singh Mani MR, Kamarul Shankar EM (2015) Attrition of hepatic damage inflicted by angiotensin II with α-tocopherol and β-carotene in experimental apolipoprotein E knock-out mice. Sci Rep 16: 18300.
22. Hedegaard M, Henriksen TB, Sabroe S, Secher NJ (1993) Psychological distress in pregnancy and preterm delivery. BMJ 307: 234-239.
23. Lyons-Ruth K, Wolfe R, Lyubchik A (2000) Depression and the parenting of young children: Making the case for early preventive mental health services. Harv Rev Psychiatry 8: 148-153.
24. Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behavior and cognition. Nat Rev Neurosci 10: 434-445.
25. Orr ST, Miller CA (1995) Maternal depressive symptoms and the risk of poor pregnancy outcome. Review of the literature and preliminary findings. Epidemiol Rev 17: 165-171.
26. Jennifer A, Ligibel MD, Wahnefried WD (2009) Diet, exercise and supplements: Guidelines for cancer survivors. American Society of Clinical Oncology, pp: 1-10.
27. Milhorat AT, Goldstone L (1965) Carrier state in muscular dystrophy of Duchenne type. JAMA 194: 130-134.
28. Blahd WH, Gassen B, Lederer M (1964) Decreased body potassium in non-dystrophic relatives of patients with muscular dystrophy. N Engl J Med 270: 197-198.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)